Research at Northwestern Qatar
Northwestern University is committed to protecting the rights and welfare of human research participants, per United States 45 CFR Part 46 Protection of Human Subjects, relevant ethical principles, and Northwestern University policy. Northwestern has an executed Master Agreement with Georgetown University in Qatar’s (GU-Q) Institutional Review Board (IRB) for the review and oversight of human research activities conducted at Northwestern Qatar.
Human Research Process Adaptation
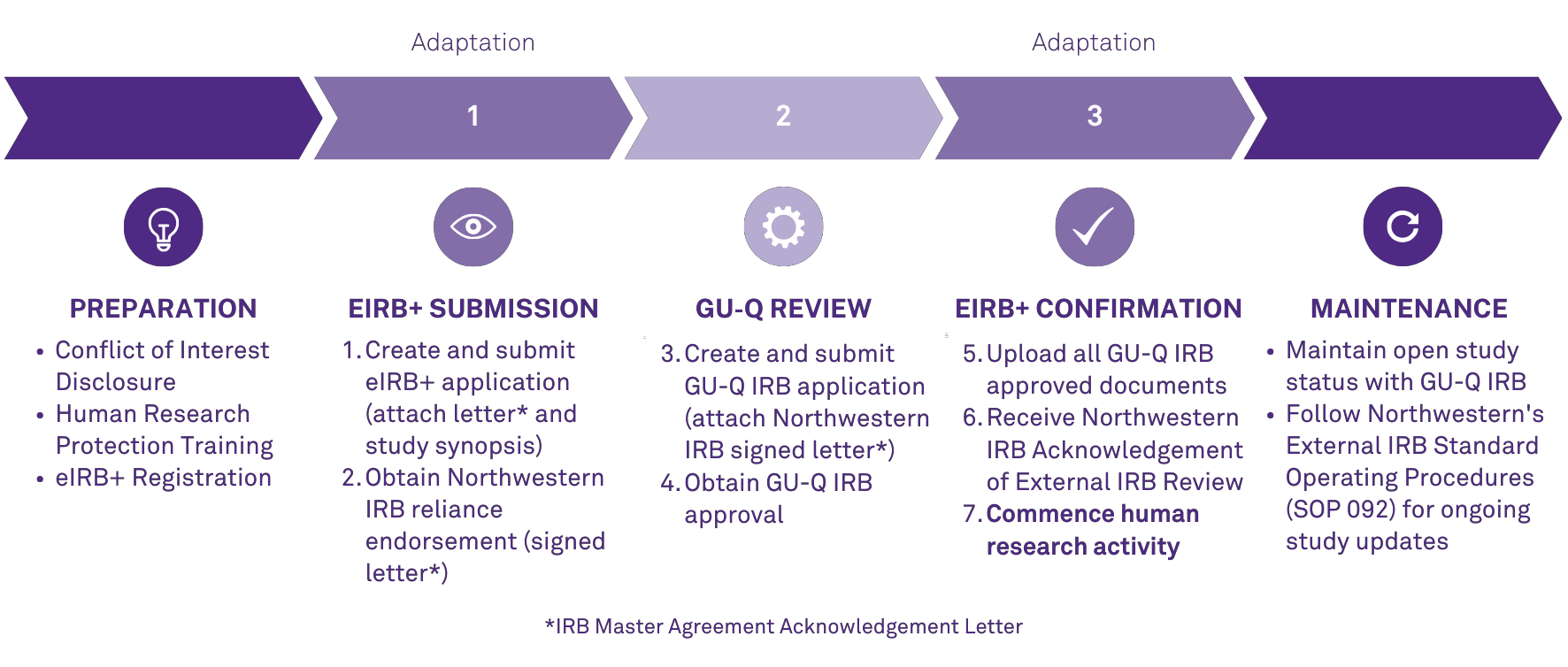
As of January 1, 2024, an adapted process will be required of all individuals involved in the conduct of human research at Northwestern Qatar. A key piece of this adaptation is utilization of eIRB+. eIRB+ is Northwestern University's electronic system for all human research. In the months leading up, the Northwestern Qatar Research Office will provide resources and training opportunities to support compliance with these mandated practices.
- Human Research Process Adaptation: Informational Quick Guide
- Share feedback here: Ongoing Support Recommendations Form
Resources
- NU-Q Tutorial for Submitting a New Study Submission in eIRB+
- GU-Q IRB Templates and Forms
- GU-Q IRB Institutional Clearance Letter HRP-739
Georgetown University in Qatar (GU-Q) IRB
All human subject research conducted at NU-Q must be prospectively reviewed and approved by an IRB. The GU-Q IRB is responsible for the review of and oversight of the conduct of human subject research that is conducted at NU-Q per our master agreement. The GU-Q IRB performs both prospective and continuing reviews of every study involving research with human subjects. These IRB reviews are done in compliance with GU and Qatar’s Ministry of Public Health (MoPH) regulations.
No human subject research may be initiated, or continued, at NU-Q without prospective approval by GU-Q IRB and acknowledgement by Northwestern IRB. Investigators at NU-Q are subject to Northwestern and GU-Q requirements.
- GU-Q: IRB Website
- GU-Q: IRB 101
- GU-Q: IRB FAQs
- GU-Q: IRB Policy Manuals
- GU-Q: Responsibilities of the Principal Investigator (PI)
MedStar IRB System
GU-Q uses the MedStar IRB System to manage their IRB review processes.
Contact Us
- Questions regarding the Human Research Process Adaptation can be sent to irbreliance@northwestern.edu
- Questions regarding PI eligibility, and gaining access to the eDisclosure platform can be sent to research@qatar.northwestern.edu
- Technical issues with eIRB+ can be sent using the eIRB+ Support Form